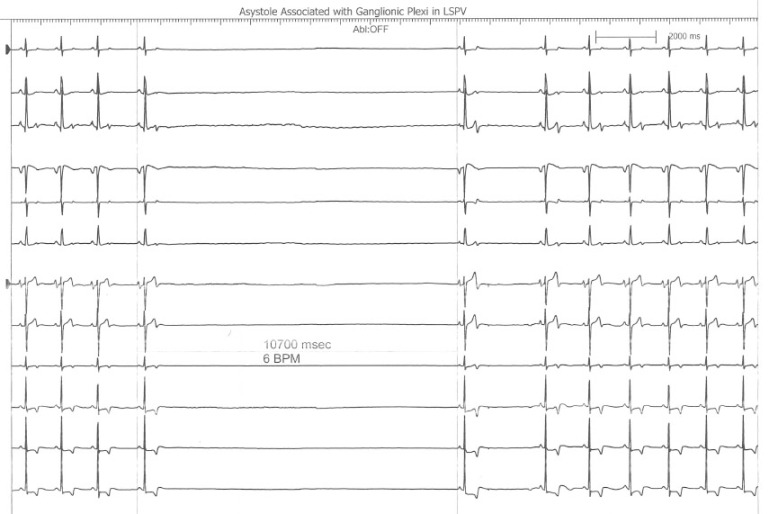
Case Description:
A 60 year old with past medical history of tobacco abuse was admitted for evaluation of chest pain without significant electrocardiogram (ECG) changes, electrolyte abnormalities or troponin elevation. Stress test revealed fixed inferolateral defects with EF44% and associated hypokinesis. Interestingly, an echocardiogram revealed an EF 55-60% with no regional wall motion abnormalities. Catheterization revealed an obstructive lesion in the PDA that had drug eluting stent successfully placed. Approximately one hour after stent placement routine ECG did not reveal any significant acute changes and patient was asymptomatic (see Figure 1). Approximately 150min after stent placement, the patient had an episode of ventricular fibrillation (VF) that required an external DC cardioversion (see Figure 2). Repeat cardiac catheterization did not reveal stent thrombosis or spasm. The patient underwent an uncomplicated single chamber defibrillator placement the following day.
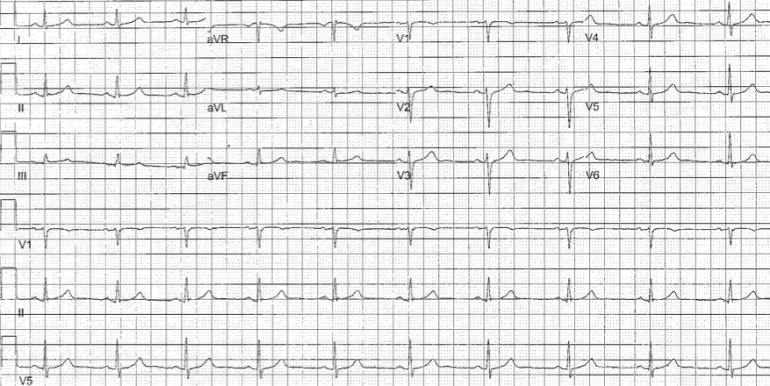
Figure 1. EKG Prior to VF Arrest

Figure 2. Telemetry Strip Showing the VF Arrest
Discussion:
VF arrest during PCI has been reported to have an incidence of 2.1% with higher incidence of VF during right coronary artery PCI. (HUA02) VF arrest during PCI is most commonly precipitated by contrast, ischemia from coronary dissection, embolism, spasm, or catheter manipulation and occurs during the cardiac catheterization. (NIS84) VF arrest after elective percutaneous coronary intervention (PCI) is uncommon. Indeed, an examination of 19,497 patients undergoing PCI revealed a 0.84% incidence of VF and no episodes of VF arrest temporally unrelated to vessel injection were reported. (ADD05) Survivors of VF arrest in the setting of myocardial infarction (MI) have similar mortality to those not experiencing VF arrest during acute MI. (DEJ09) In contrast, mortality in survivors of in-hospital cardiac arrest has been reported as high as 47% during a median followup of 1.3 years. (HEL11) It is unclear if the mechanism of VF arrest in our patient is secondary to PCI or rather a primary VF arrest. There is a prior report of delayed three vessel coronary spasm in a patient receiving paclitaxel drug-eluting stents however, coronary spasm was demonstrated on repeat catheterization in that report. (KIM05) Our patient did not report any ischemic symptoms preceding his VF arrest (though his EKG had subtle ST changes suggesting possible ischemia) nor did his repeat catheterization reveal vessel thrombosis, spasm, or dissection. Additionally, peri- and post-procedural myocardial injury from slow coronary flow, microvascular embolization, and elevated levels of troponin causing reperfusion tissue damage and cardiac dysfunction leads to worse long-term prognosis than those without myocardial injury (ISH08); our patient did not have significantly elevated pre- or post-procedure troponin levels. The time course of ischemia-induced reperfusion changes is likely less than 30minutes based upon prior experimental models of ischemia. (WIL08) Five minutes of coronary artery occlusion avoids increased risk of ventricular arrhythmias in animals and 30 minutes is appropriate for adequate reperfusion. (DAV81,DAV82,RUF79,WIL08) When the left anterior descending artery is transiently occluded in dogs, there is an initial (t=0-2minutes) small decrease in peak R wave amplitude and conduction velocity followed by a large increase in these indices over the ensuing 1-2minutes. (HOL76) There is a rapid return to baseline when occlusion is released and reperfusion occurs. This biphasic response has also been documented in dogs undergoing circumflex artery occlusions lasting 5minutes. (DAV81,DAV82) However, Ruffy et al (RUF79) found that LAD occlusions for 5 minutes in the dog resulted in a decrease in electrogram R wave amplitudes with no biphasic response. The progressive decrease in R wave amplitude (with the subsequent increase in amplitude) has been demonstrated in isolated rabbits hearts during global ischemia over 10 minutes (KAB89), isolated pig hearts during LAD occlusions for 5 minutesJAN86, and humans subject to 60minutes of unresolved ischemia. (VAI94) Of note, these electrical alterations were rapidly reversible upon reperfusion. (HOL76,RUF79,KAB89,JAN86) In summary then, our patient experienced a VF arrest 150min after elective PCI without conclusive evidence of procedural-related ischemia and well outside the conventional 30min window of reperfusion electrical alterations seen in experimental models. The role of defibrillator implantation as secondary prevention in patients like this is unclear.
References:
HUA02 Huang JL, Ting C-T, Chen Y-T, Chen S-A, “Mechanisms of ventricular fibrillation during coronary angioplasty: increased incidence for the small orifice caliber of the right coronary artery,” International Journal of Cardiology, Volume 82, Issue 3 (March 2002), pp. 221-228.
NIS84 Nishimura RA, Holmes DR Jr, McFarland TM, Smith HC, Bove AA, “Ventricular arrhythmias during coronary angiography in patients with angina pectoris or chest pain syndromes,” Am J Cardiol. V. 53, No. 11 (June 1984), pp. 1496-9.
ADD05 Addala S. Kahn JK, Moccia TF, Harjai K, Pellizon G, Ochoa A, O’Neill WW, “Outcome of Ventricular Fibrillation Developing During Percutaneous Coronary Interventions in 19,497 Patients Without Cardiogenic Shock,” Am J Card, V. 96 (2005), pp. 764-765.
HEL11 Helton TJ, Nadig V, Subramanya SD, Menon V, Ellis SG, Shishehbor MH, “Outcomes of cardiac catheterization and percutaneous coronary intervention for in-hospital VT/VF cardiac arrest, Catheter Cardiovasc Interv, [Epub ahead of print], (July 6, 2011).
DEJ09 DeJong JSSG, Marsman RFHenriques JPS, Koch KT, de Winter RJ, Tanck MWT, Wilde AAM, Dekker LRC, “Prognosis among survivors of primary ventricular fibrillation in the percutaneous coronary intervention era,” Am Heart J, V. 158, No. 3 (September 2009), pp. 467-472.
KIM05 Kim JW, Park CG, Seo HS, Oh DJ, “Delayed severe multivessel spasm and aborted sudden death after Taxus stent implantation,” Heart, V. 91, No. 2 (Feb 2005 Feb), e15.
ISH08 Ishiia H, Amanoa T, Matsubarabv T, Murohara T, “Pharmacological Prevention of Peri-, and Post-Procedural Myocardial Injury in Percutaneous Coronary Intervention,” Current Cardiology Reviews, V. 4. No. 3 (August 2008), pp. 223-230.
WIL08 Williams JL, Mendenhall GS, Saba S, “Effect of Ischemia on Implantable Defibrillator Intracardiac Shock Electrograms,” J Cardiovasc Electrophysiology, Vol. 19, No. 3 (March 2008), pp. 275-281.
DAV81 David D, Naito M, Chen CC, Michelson EL, Morganroth J, Schaffenburg M, “R-wave Amplitude Variations During Acute Experimental Myocardial Ischemia: An Inadequate Index for Changes in Intracardiac Volume,” Circulation, V. 63, No. 6 (June 1981), pp. 1364-1370.
DAV82 David D, Naito M, Michelson EL, Watanabe Y, Chen CC, Morganroth J, Schaffenburg M, Blenko T, “Intramyocardial Conduction: A Major Determinant of R-wave Amplitude During Acute Myocardial Ischemia,” Circulation, V. 65, No. 1 (January 1982), pp. 161-167.
RUF79 Ruffy R, Lovelace DE, Mueller TM, Knoebel SB, Zipes DP, “Relationship between Changes in Left Ventricular Bipolar Electrograms and Regional Myocardial Blood Flow during Acute Coronary Artery Occlusion in the Dog,” Circulation Research, V. 45, No. 6 (December 1979), pp. 764-770.
HOL76 Holland RP, Brooks H, “The QRS Complex during Myocardial Ischemia,” Journal Clinical Investigation, V. 57 (March 1976), pp. 541-550.
KAB89 Kabell G, “Ischemia-Induced Conduction Delay and Ventricular Arrhythmias: Comparative Electropharmacology of Bethanidine Sulfate and Bretylium Tosylate,” Journal Cardiovascular Pharmacology, V. 13, No. 3 (1989), pp. 471-482.
VAI94 Vaitkus PT, Miller JM, Buxton AE, Josephson ME, Laskey WK, “Ischemia-induced changes in human endocardial electrograms during percutaneous transluminal coronary angioplasty,” American Heart Journal, V. 127, No. 6 (June 1994), pp. 1481-1490.
JAN86 Janse MJ, “Electrophysiology and electrocardiology of acute myocardial ischemia,” Can J Cardiology, Supp. A (July 1986), pp. 46A-52A.