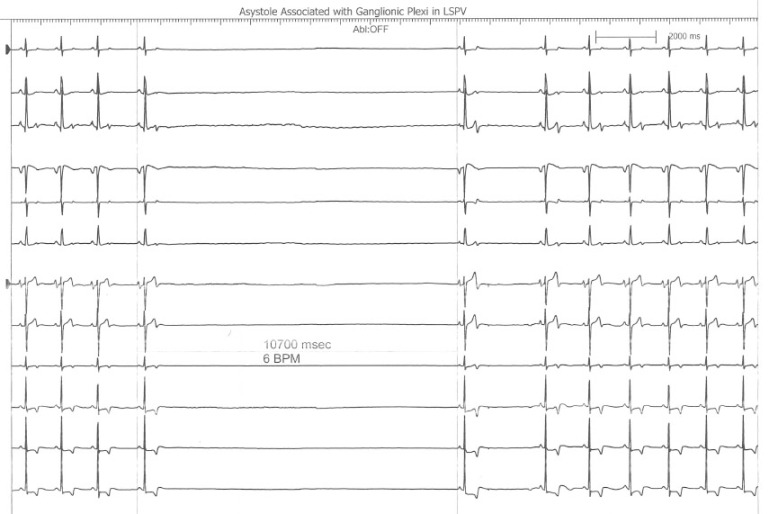
This is an interesting finding observed during a recent atrial fibrillation ablation performed in our Heart Rhythm Center. The ablation paradigm has been previously described [1] and consists of a pulmonary venous antrum isolation using entrance and exit block criteria guided by intra left atrial radial intracardiac echocardiography (ICE). During the initial antrum encircling lesion asystole developed (see following figure), ablation was stopped, and sinus rhythm recovered within 10seconds.
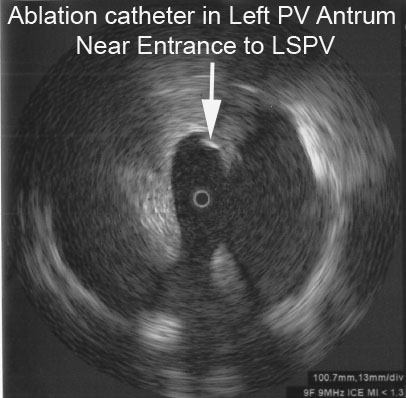
The following radial ICE image demonstrates the ablation catheter location in the superior aspect of the left pulmonary venous antrum near the left atrial appendage.
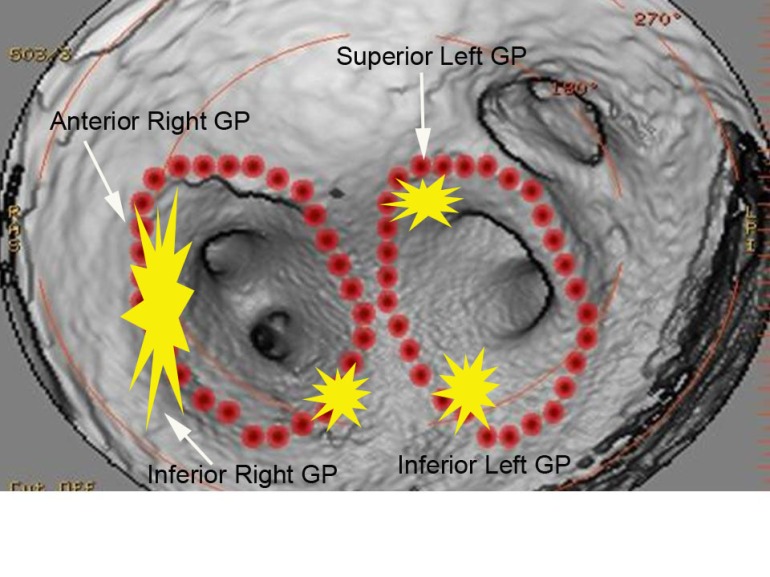
Bradycardia is often seen during atrial fibrillation ablations when proximate to autonomic ganglionic plexi. [2] I routinely see fluctuations in basal sinus rate during pulmonary venous antrum ablations but this was more dramatic than the sinus rate changes I usually observe. This location as seen on the intra left atrial radial ICE shot is slightly more anterior than the left superior ganglionic plexus is usually expected. The following figure shows a CT reconstruction of the posterior left atrium and pulmonary venous antra. The red dots depict a typical venous antrum ablation lesion set and the yellow areas denote the approximate locations of the ganglionic plexi. [3] Discontinuation of ablation led to quick restoration of sinus rhythm and repeat ablation near this location to finalize lesion set did not result in repeat asystole or significant fluctuations in sinus rate.
Another possible explanation for this finding is acute sinus node dysfunction (from damage to the sinus node artery, SNA) during ablation in the anterior left atrium. Chugh et al present an excellent review of coronary arterial injury during ablation of atrial fibrillation. [4] Though there was no obvious PR prolongation prior to the pause suggesting an autonomic effect, there was also no obvious sinus tachycardia or acceleration serving as a “harbinger of impending [sinus node] dysfunction.” Though the SNA arises from the RCA in two-thirds of patients, the remainder of SNA arise from an early branch of the circumflex which “passes superiorly and to the right of the LAA and courses over the anterior LA before terminating at the cavoatrial junction.” Less commonly, the SNA branches off a more distal portion of the circumflex and ascends in the lateral ridge between the appendage and the left pulmonary veins. The patient had an uneventful post-ablation recovery.
References:
2 Pappone C, et al “Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation,” Circulation, V. 109 (2004), p. 327.
3 Katritsis DG et al, “Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation A Randomized Clinical Trial,” JACC, V. 62 (December 2013), pp. 2318–25.
4 Chugh A et al, “Manifestations of coronary arterial injury during catheter ablation of atrial fibrillation and related arrhythmias,” Heart Rhythm, V. 10, No. 11 (November 2013), pp. 1638-1645.