Artificial composites, metamaterials, can be used as implantable biosensors. Silk is not only tough but is very compatible with most tissue surfaces in the human body. This combined with highly conductive metals (such as gold, silver, and copper) can be tweaked on a nanoscale level and combined with other materials to respond to frequencies in the terahertz range. As it turns out, proteins in the body resonate at specific frequencies within the terahertz range, making them easy to identify with the right type of biosensors.
Month: May 2012
Heart Rhythm Management and Drug Delivery via Implantable Biosensors
Your Next Prescription Might Be For A Microchip – Forbes.
Drug delivery via implantable biosensors is the next generation of devices that can be used to help heart rhythm disorders. Our current implantable loop recorders allow us to track heart rhythm disorders and link symptoms to arrhythmias. Implantable biosensors will combine this monitoring ability with automated drug delivery.
Pacemaker Implantation in the Extreme Elderly

There are scant data for pacemaker implant complications and readmission rates in the extreme elderly (age≥80 years) despite their common use in this population. We performed a retrospective chart review of consecutive patients (n=149, age≥80 years) who underwent pacemaker implantation at our community hospital Electrophysiology program from July 2008 through June 2010. Single-, dual-, and biventricular-chamber pacemakers and generator changes were included for analysis; cardioverter-defibrillator devices, temporary pacemakers, and loop recorders were excluded. Standard procedures for implantation were used. Major complications defined as death, cardiac arrest, cardiac perforation, cardiac valve injury, coronary venous dissection, hemothorax, pneumothorax, transient ischemic attack, stroke, myocardial infarction, pericardial tamponade, and arterial-venous fistula. Minor complications defined as drug reaction, conduction block, hematoma or lead dislodgement requiring reoperation, peripheral embolus, phlebitis, peripheral nerve injury, and device-related infection.
The overall mean age of implantation was 86 years. There were no intraprocedural complications. There was one major in-hospital (0.7%) and one minor in-hospital complication (0.7%). Within 30 days of implant, there was an overall 5.4% rate of complications; 4 minor (2.7%) and 4 major (2.7%). There was a 30d cardiovascular-attributable mortality of 0.7% and an all-cause mortality of 2%. There was a 5.4% rate of readmission within 30days of implantation.
Our report of pacemaker implantations in the extreme elderly reveals rates of implant complications comparable to data from younger patient populations while experiencing a higher 30day all-cause mortality (that may be attributable to elevated all-cause mortality rates in this age-group).
High Frequency Jet Ventilation During Ablation of Supraventricular and Ventricular Arrhythmias
High-frequency jet ventilation (HFJV) is used to decrease respiratory motion during atrial fibrillation ablations; however, the patient safety and efficacy of HFJV has not been evaluated during routine electrophysiology (EP) studies with radiofrequency ablation. This is a retrospective chart review of consecutive patients who underwent EP studies and ablations for supraventricular and ventricular arrhythmias while using HFJV. Any EP studies performed using HFJV where ablation was attempted were included for analysis; EP studies where no ablation was performed were not included. Patients underwent induction of general anesthesia with endotracheal intubation using intermittent positive pressure ventilation with sevoflurane in the EP laboratory prior to vascular access. HFJV was then provided by a commercial system with initial settings: ventilation rate at 100 cycles/min and driving pressure at 20–25 psi. Total intravenous anesthesia was then provided with dexmedetomidine and propofol as well as fentanyl and rocuronium titrated to bispectral index (Bis) score <60. The overall mean age of patients (n=72) was 55+/-18 years (ranges 18–84 years). The mean creatinine (mg/dl) was 1.0+/-0.3, the mean ejection was 0.58+/-0.08, and mean post-EP study length of stay was 1.4+/-0.9 days (range 1–5 days). There were no intraprocedural or major complications. There was a 6.9% rate of minor complications (n=5). There was a 97.2% overall ablation success rate (70 of 72 ablations). Ablations were successful in all subjects except for one left atrial flutter and one right atrial tachycardia. Only one of 72 (1.4%) procedures required discontinuation of general anesthesia and HFJV to induce arrhythmia (right ventricular outflow tract ventricular tachycardia). No patient experienced procedural awareness and the mean Bis score was 40+/-5.3. This report provides further evidence the routine use of jet ventilation in the electrophysiology laboratory is safe, well tolerated, and efficacious, with ablation success rates similar to traditional sedation/ventilation techniques with a variety of arrhythmias.
For Full Study Please See: http://www.innovationsincrm.com/cardiac-rhythm-management/2011/november/157-high-frequency-jet-ventilation
Implementation of a Highly-Performing Electrophysiology Device Implant Program: Is There a Role for Niche Hospitals?
Background: Single-center reports on patient demographics and early (<6 weeks) device complication rates in academic hospitals are scant and non-existent for non-academic community hospital electrophysiology (EP) programs. Objective: The objective of our study was to examine the demographics, complications, re-admissions, and accessibility of care in a community EP program to add to the body of knowledge of ‘real-world’ defibrillator implant complications. Methods: Two hundred and fifty consecutive patients who underwent device implantation by a single electrophysiologist in a new non-academic community hospital EP program starting from its inception in July 2008 were included for analysis. Standard procedures for implantation were used. Pacemakers, defibrillators, and generator changes were included; temporary pacemakers were excluded. Major complications were defined as in-hospital death, cardiac arrest, cardiac perforation, cardiac valve injury, coronary venous dissection, hemothorax, pneumothorax, transient ischemic attack, stroke, myocardial infarction, pericardial tamponade, and arteriovenous fistula. Minor complications were defined as drug reaction, conduction block, hematoma or lead dislodgement requiring re-operation, peripheral embolus, phlebitis, peripheral nerve injury, and device-related infection. Results: This community cohort had similar ejection fractions but was older with worse kidney function than those studied in prior reports. There was one major early complication (0.4%) and seven minor early complications (2.8%). Left ventricular lead placement was successful in 64 of 66 patients (97%). Conclusions: This is the first community-hospital based EP program to examine device implant demographics and outcomes, and revealed an elderly, ill population with lower overall rates of complications than seen in national trials and available reports from single non-community centers. Contrary to current perceptions, these data suggest that community centers may subselect an elderly, ill patient population and can provide high-quality, cost-effective, and more accessible care.
For More Details Please See:
American Heart Hospital Journal: http://www.touchbriefings.com/ebooks/A1oe7m/ahhj81/resources/35.htm
EP Lab Digest: http://www.eplabdigest.com/articles/Implementation-a-Highly-Performing-Electrophysiology-Device-Implant-Program-Is-There-a-Role.
Implantable Biosensors and Future Role in Electrophysiology
Implantable loop recorders (ILR) are used for long-term arrhythmia monitoring inpatients that have had syncope or cryptogenic strokes (possible from atrial fibrillation). There are two primary models of loop recorders: St. Jude Medical’s Confirm (http://www.sjmprofessional.com/Products/US/Implantable-Cardiac-Diagnostics/SJM-Confirm-Implantable-Cardiac-Monitor.aspx) and Medtronic’s Reveal (http://www.medtronic.com/for-healthcare-professionals/products-therapies/cardiac-rhythm/cardiac-monitors-insert/reveal-dx-and-reveal-xt-insertable-cardiac-monitors-icms/index.htm).
ILR are placed just under the skin in the left chest and are able to record a patient’s heart rhythm. It helps to diagnose the cause of syncope (fainting) or any number of heart rhythm disorders. Initially, doctors try to diagnose heart rhythm disorders with monitors that are worn for 48hours to 2-4weeks; however, an arrhythmia that may only occur every few months will not be detected by a brief snapshot in a patient’s life. ILR generally last 2-3 years before the battery wears out.
The exciting future of implantable monitors is not simply heart rhythm diagnosis. Recently, the company MicroCHIPS announced results of the first human clinical trial of an implantable, wireless microchip drug delivery device (http://www.mchips.com/technology.html). This type of implantable device can allow automated drug delivery over a period of several years. One can imagine a device that can track a person’s heart rate and dose medication to permit precise control of heart during an abnormal heart rhythm.
Three Dimensional Reconstruction of Left Atrium
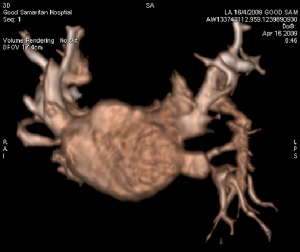
The performance of complex cardiac procedures, such as advanced defibrillator placement, structural heart interventions, or arrhythmia ablation, is facilitated by the visualization of 3D anatomy. Providing 3D views of internal body structures and interventional devices in one image, this state-of-the-art system assists physicians in diagnosis, surgical planning, interventional procedures and treatment follow-up. It permits better management of structural heart disease, streamlines interventional procedures, and minimizes radiation dose to physicians, staff and patients by selecting working views without fluoroscopy. Patients can undergo 3D angiography of the coronary sinus to guide a biventricular defibrillator implantation with a left ventricular pacemaker lead. Patients can also undergo 3D angiography of the left atrium and pulmonary veins to plan an atrial fibrillation arrhythmia ablation.


